Abstract
BACKGROUND: Relapse is the major cause of death in patients with acute myeloid leukemia after allogeneic hematopoietic cell transplant (HCT). Measurable residual disease (MRD) detected by multiparameter flow cytometry (MFC) before and after transplant (peri-transplant) is a strong, independent risk factor for post-transplant relapse. In a previous study, we demonstrated pre-transplant MRD detected by MFC conveys high-risk of relapse, irrespective of whether the MRD is cleared at day 28 after transplant. However, the sensitivity of MFC based MRD test appeared insufficient to identify all high-risk patients, as post-transplant residual disease was not detected in some patients who rapidly relapsed. We hypothesized that the significance of post-transplant MRD might be better demonstrated by using more sensitive MRD testing in a relatively homogenous disease population. In this study, we investigated changes in MRD detected before and after transplant by MFC and next-generation sequencing (NGS) in patients with NPM1 -mutated AML (NPM1M).
PATIENTS AND METHODS: Patients older than 18 years of age who underwent first HCT for NPM1 mutated AML were eligible for this study if they were in complete remission before transplant as determined by morphology and achieved successful engraftment (within 35 days). Bone marrow MRD was assessed within 6 weeks prior to the transplant and at day 28 (+/- 7 days) after the transplant. MFC MRD testing was performed using 10-color flow cytometry with an 18-antibody panel; the leukemic blasts were identified using a difference-from-normal approach. NPM1M was detected using NGS amplicon sequencing targeting NPM1 exon 12 with an average of 800,000x fold coverage. The technical limit of NPM1M detection was 0.002% (0.004% of NPM1M -positive cells). MRD was empirically divided into three levels: MRDHi (MRD detected by MFC or NPM1M >=0.02%), MRDLo (MFC negative and NPM1M >=0.002% but <0.02%), and MRDNeg (MFC negative and NPM1M negative). In addition, post-transplant MRD increase was defined as MRD conversion from negative to positive detected by either MFC or NGS; and post-transplant MRD decrease was defined as MRD conversion from positive to negative detected by MFC or NGS of more than one log decrease in NPM1M . Peri-MRD high combined pre-transplant MRDHi and post-transplant MRD increase. Peri-MRD low combined peri-transplant MRDNeg and pre-transplant MRDLo without post-transplant increase. The primary end point of this analysis was relapse-free-survival (RFS) estimated using the Kaplan-Meier method and multivariate Cox regression.
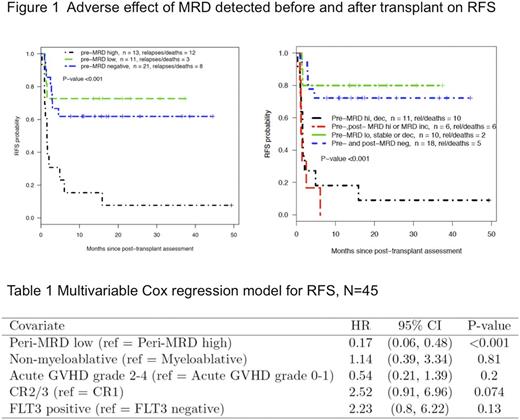
RESULT: Peri-transplant MRD was measured using the MFC and NGS assays in 45 patients with median age 58 years (range 22-79). The median follow up in patients remaining in remission was 18 months (range 9-50). FLT3-ITD was positive in 25 of the 45 patients. At the time of HCT, 24 patients were in CR1, 19 in CR2 and 2 in CR3. Thirty patients received myeloablative conditioning versus 15 who received nonmyeloablative conditioning. Before transplant, 13 patients had MRDHi (9 detected by MFC), 11 had MRDLo, and 21 were MRDNeg. Post transplant, 9 patients had MRDHi (5 detected by MFC), 7 had MRDLo, and 29 were MRDNeg. The patients with pre-transplant MRDHi had significantly adverse RFS as compared to patients with MRDLo (HR=6.2, p=0.006) or MRDNeg (HR=4.8, p<0.002) (Figure 1a). Taking post-transplant MRD into consideration, patients experiencing post-transplant MRD increase or having MRDHi before transplant, irrespective of a post-transplant MRD decrease, had significantly shorter RFS than patients having pre-transplant MRDLo without post-transplant MRD increase or being peri-transplant MRD negative (HR=7.7, p<0.001)(Figure 1b). In multivariate analysis, peri-MRD high was the most significant risk factor for adverse RFS, whereas the intensity of the conditioning regimen, aGVHD status, disease status and FLT3-ITD were not significant after adjustment for peri-transplant MRD. (Table 1).
CONCLUSION: High-level pre-transplant MRD and post-transplant MRD increase convey an extremely high risk of post-transplant relapse in patients with NPM1 mutated AML. MFC testing can identify a subset of high-risk patients prior to transplant, but more sensitive MRD detection by NGS, especially after marrow engraftment, may help to identify additional high-risk patients who may benefit from pre-emptive treatment.
Walter: Aptevo Therapeutics: Research Funding; ADC Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal